Oregon Patent of the Month – February 2024
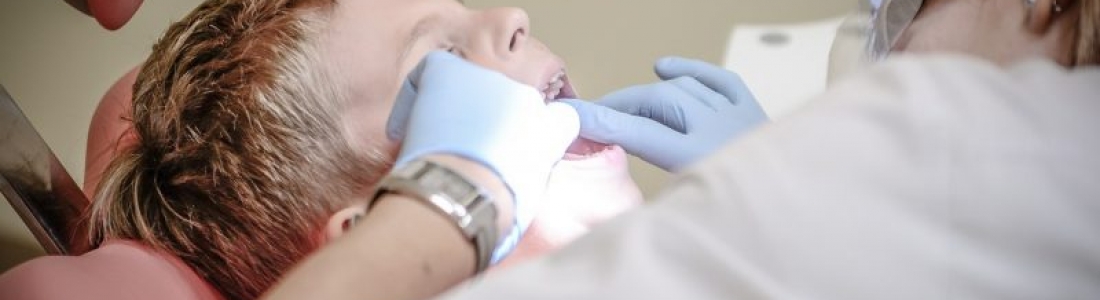
TriAgenics, Inc., a pioneering force in dental innovation, has unveiled a groundbreaking solution that challenges the conventional approach to wisdom tooth extraction. The Zero3™ Tooth Bud Ablation (TBA) procedure and system offer a revolutionary alternative to the traditional, invasive extraction methods, addressing the challenges and risks associated with wisdom tooth removal.
The core of the TriAgenics invention lies in a sophisticated ablation method that employs a virtual stent and a movement-sensored ablation probe tip. The procedure involves providing a virtual stent along with a specialized ablation probe equipped with movement sensors, a combination that transforms the extraction process. By incorporating at least one volume scan, virtual surgical guide angles, and virtual stops, TriAgenics ensures precise guidance and limitation of the ablation probe’s depth.
One of the key features of this innovation is the real-time feedback displayed on a monitor, presenting representations of the ablation probe, surgical guide angles, and stops. This provides dental professionals with an unprecedented level of control and visualization during the procedure.
TriAgenics emphasizes the potential for early intervention, addressing tooth bud formation in children aged 6 to 10, significantly reducing the risks and complications associated with later-life extractions. The traditional approach of prophylactic extraction of wisdom teeth, often performed during adolescence, is challenged by TriAgenics’ tooth bud ablation, eliminating the need for invasive surgery in adulthood.
The Tooth Bud Ablation procedure’s benefits extend beyond reducing the economic burden on patients and minimizing the risks associated with delayed interventions. TriAgenics envisions a paradigm shift in dental care, promoting a proactive and patient-friendly approach to managing third molars. This innovative solution offers a unique blend of technology, precision, and real-time feedback to transform the landscape of wisdom tooth management.
TriAgenics is currently still pursuing final FDA approval. Once granted, the company intends for Zero3 patient-specific surgical kits to be delivered directly to the practitioner to perform the 60-90 second 3TBA treatment. Their technology has been proven in animal studies with no observed adverse outcomes other than one post-op infection.
Are you developing new technology for an existing application? Did you know your development work could be eligible for the R&D Tax Credit and you can receive up to 14% back on your expenses? Even if your development isn’t successful your work may still qualify for R&D credits (i.e. you don’t need to have a patent to qualify). To find out more, please contact a Swanson Reed R&D Specialist today or check out our free online eligibility test.
Who We Are:
Swanson Reed is one of the U.S.’ largest Specialist R&D tax advisory firms. We manage all facets of the R&D tax credit program, from claim preparation and audit compliance to claim disputes.
Swanson Reed regularly hosts free webinars and provides free IRS CE and CPE credits for CPAs. For more information please visit us at www.swansonreed.com/webinars or contact your usual Swanson Reed representative.