Iowa Patent of the Month – November 2023
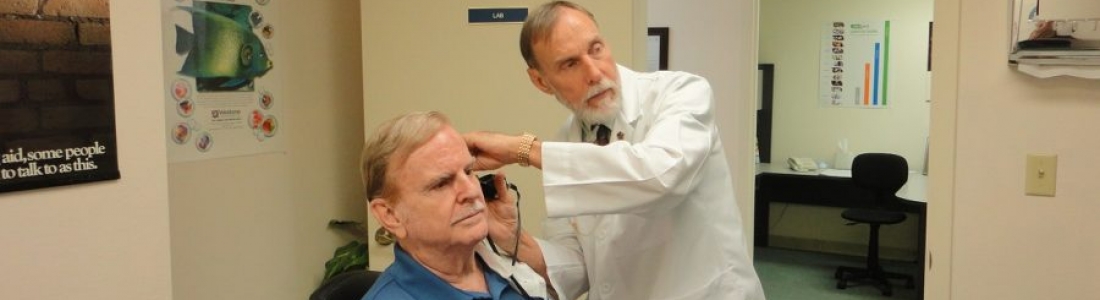
Ear infections, particularly acute otitis media (AOM), present challenges in accurate diagnosis. The subjective nature of conventional methods and the potential for error in interpretation have historically led to an over-diagnosis of ear infections. However, the landscape of medical diagnostics is rapidly transforming, and Digital Diagnostics, Inc. is pioneering the future of healthcare by developing an autonomous diagnostic approach that doesn’t rely on human intervention.
By leveraging cutting-edge AI and machine learning models, it accurately assesses, diagnoses, and prescribes therapy for ear infections, significantly reducing the burden caused by over-diagnosis in the current systems.
The technology uses a wealth of patient data—medical history, vitals, and even patient-provided information about their condition. Biomarker features extracted from ear measurements are synthesized with this data, creating an input set for the machine learning model. These biomarker features, including image and acoustic markers, hold critical information about the ear’s condition, such as anatomical structures and responses to pressure stimuli. It’s this depth of information that’s revolutionizing the diagnostic landscape.
The technology learns from extensive training examples, crafting a diagnostic model that becomes increasingly accurate. As a result, it outputs probability-based diagnoses, indicating the likelihood of ear infections, empowering healthcare professionals with reliable insights.
What’s particularly transformative is the direct impact on treatment. The system not only diagnoses but also prescribes therapy based on the diagnosis. This includes leveraging expert systems, employing rules, and probability models to determine the most effective course of action for the patient.
This autonomous diagnostic approach marks a seismic shift in healthcare. Digital Diagnostics’ success with their flagship product, LumineticsCore™ (previously IDx-DR) , which diagnoses diabetic retinopathy, underscores their ability to democratize healthcare by delivering autonomous, efficient, and equitable diagnostic platforms.
By transforming diagnostics, Digital Diagnostics is paving the way for a new standard of care, driving better patient outcomes and revolutionizing the accessibility and affordability of quality healthcare.
Are you developing new technology for an existing application? Did you know your development work could be eligible for the R&D Tax Credit and you can receive up to 14% back on your expenses? Even if your development isn’t successful your work may still qualify for R&D credits (i.e. you don’t need to have a patent to qualify). To find out more, please contact a Swanson Reed R&D Specialist today or check out our free online eligibility test.
Who We Are:
Swanson Reed is one of the U.S.’ largest Specialist R&D tax advisory firms. We manage all facets of the R&D tax credit program, from claim preparation and audit compliance to claim disputes.
Swanson Reed regularly hosts free webinars and provides free IRS CE and CPE credits for CPAs. For more information please visit us at www.swansonreed.com/webinars or contact your usual Swanson Reed representative.