Nebraska R&D Tax Credit Filing Instructions
To claim the Research and Development (R&D) tax credit in Nebraska, businesses must first incur research and experimental (R&D) expenditures within the state, as defined by Internal Revenue Code (IRC) § 174, and generally claim the federal R&D tax credit. The Nebraska R&D tax credit is typically a percentage of the federal credit allowed. Businesses then claim the credit on their Nebraska tax return by completing Form 3800N, Nebraska Incentives Credit Computation, and its accompanying worksheet, Form 3800N Worksheet RD, Research Tax Credit Worksheet. This form and worksheet are submitted along with the taxpayer’s Nebraska income tax return. The credit can be used to reduce income tax liability or, in some cases, obtain a refund of state sales and use taxes paid. Businesses that conduct R&D activities on a college or university campus in Nebraska may qualify for an enhanced credit rate. It is also important to note that businesses claiming the credit must electronically verify the work eligibility status of all newly-hired employees in Nebraska during the tax year in which the credit is claimed.
Nebraska Patent of the Year – 2024/2025
Ting Therapeutics LLC has been awarded the 2024/2025 Patent of the Year for their groundbreaking approach to preventing and treating hearing loss. Their invention, detailed in U.S. Patent No. 11857551, titled ‘Methods for the prevention and treatment of hearing loss’, introduces a novel method for protecting ear cells from damage caused by chemotherapy and noise exposure.
This patented method involves the use of specific compounds to treat and prevent hearing impairments. The compounds work by targeting and protecting the sensitive cells in the inner ear that are susceptible to damage from various sources. By preserving these cells, the method aims to maintain hearing function and prevent the onset of hearing loss.
Ting Therapeutics’ innovation addresses a significant health concern, as acquired hearing loss due to chemotherapy or noise exposure is a major issue. Current treatments are limited, and there are no FDA-approved drugs for the prevention or treatment of such hearing loss. This new approach offers hope for individuals at risk, providing a potential therapeutic option to safeguard hearing health.
With this advancement, Ting Therapeutics LLC is paving the way for new treatments that could significantly improve the quality of life for those affected by hearing loss, marking a significant step forward in medical innovation.
Study Case
Nut & Co. was started in 1988 to design and build equipment to fulfill the needs of the pecan grower and sheller.
The owner of Nut & Co. began his pecan career in 1972. Since the very first day an effort to help build the industry stronger has always been in the forefront of the company. Designing and manufacturing high-quality, reliable equipment has helped build a company that each year continues to be a leader in the industry.
Nut & Co. incurred qualified research expenses under IRC Section 41 relating to the design and development of various cultivation, support, processing and separation equipment during the 2013-2016 fiscal years. Its biggest R&D project was the development of the Aspirator System.
The Aspirator System was designed in 2012 to fit both small and larger shellers. At the time, the industry used blowers to separate shell from pecan meat. This process was inefficient and produced a lot of dust within the plant. The aspirator system was designed to use suction methods instead of blowing. This kept the plant clean and accomplished the remarkable task of removing the shell from the pecan meat.
In order to qualify for the Research and Development Tax Credit, Nut & Co. needed to determine the eligibility of its proposed R&D activities. The qualified research must meet four main criteria, known as the Four-Part Test. Nut & Co.’s qualified R&D activities included the following.
Design and development of a series of prototypes to achieve the technical objectives (design of the aspirator system).
Nut & Co.’s hypothesis for this activity stated that it was possible to develop a revolutionary piece of shelling equipment that could remove the meat from the pecan shell by using a suction method instead of the normal blowing method.
The experiments that Nut & Co. conducted in the design phase predominantly entailed conceptual engineering drawings, mathematical calculations and testing of different materials. These experiments could only be proven effective or ineffective in the prototype development and testing phase. Following the experiments in that phase, during which the product was built and tested in various applications, the design was modified and retested until the desired outcome was achieved.
Trials and analysis of data to achieve results that can be reproduced to a satisfactory standard (development and testing of the aspirator system).
The main objective for this activity stated that with improved knowledge of the intrinsic factors related to the extraction of pecans from their shells, it was possible to identify mechanisms for improving the current shelling process.
Details of this experiment included testing of different materials ultimately concluding that stainless steel wasp, off-fall tanks and galvanized piping needed to be used for the system to ensure efficiency, accuracy and safety.
Background research to evaluate current knowledge gaps and determine feasibility (background research of the development of the aspirator system).
Prior to 2013, the shelling equipment existing on the market was cumbersome and expensive. Thus, besides the lack of comparable solutions available, the outcomes of activities in this research could not have been known or determined in advance due to a number of specific technical challenges.
Nut & Co.’s eligible R&D activities during this phase of experimentation included:
- Literature search and review, including maintaining up-to-date knowledge on relevant certification and standards.
- Consultation with industry professionals and potential customers to determine the level of interest and commercial feasibility of the product.
- Preliminary equipment and resources review with respect to capacity, performance and suitability for the project.
- Consultation with key component/part/assembly suppliers to determine the factors they considered important in the design and to gain an understanding of how the design needed to be structured accordingly.
The background research conducted by Nut & Co. was directly related to the main objective of designing the aspirator system, therefore qualifying as R&D.
Ongoing analysis of customer or user feedback to improve the prototype design (feedback R&D of the aspirator system).
Nut & Co.’s eligible R&D activities for this phase of its project included:
- Ongoing analysis and testing to improve the efficiency and safety of the product.
- Ongoing development and modification to interpret the experimental results and draw conclusions that served as starting points for the development of new hypotheses.
- Commercial analysis and functionality review.
These activities were necessary to evaluate the performance capabilities of the new design in the field and to improve any flaws in the design, therefore qualifying as R&D.
Qualified research consists of research for the intent of developing new or improved business components. A business component is defined as any product, process, technique, invention, formula, or computer software that the taxpayer intends to hold for sale, lease, license, or actual use in the taxpayer’s trade or business.
The Four-Part Test
Activities that are eligible for the R&D Credit are described in the “Four-Part Test” which must be met for the activity to qualify as R&D.
- Permitted Purpose: The purpose of the activity or project must be to create new (or improve existing) functionality, performance, reliability, or quality of a business component.
- Elimination of Uncertainty: The taxpayer must intend to discover information that would eliminate uncertainty concerning the development or improvement of the business component. Uncertainty exists if the information available to the taxpayer does not establish the capability of development or improvement, method of development or improvement, or the appropriateness of the business component’s design.
- Process of Experimentation: The taxpayer must undergo a systematic process designed to evaluate one or more alternatives to achieve a result where the capability or the method of achieving that result, or the appropriate design of that result, is uncertain at the beginning of the taxpayer’s research activities.
- Technological in Nature: The process of experimentation used to discover information must fundamentally rely on principles of hard science such as physical or biological sciences, chemistry, engineering or computer science.
What records and specific documentation did Nut & Co. keep?
Similar to any tax credit or deduction, Nut & Co. had to save business records that outlined what it did in its R&D activities, including experimental activities and documents to prove that the work took place in a systematic manner. Nut & Co. saved the following documentation:
Project records/ lab notes
Innovation Log
Conceptual sketches
Design drawings
Background research
Records of changes
Testing protocols
Results of records of analysis from testing/trial runs
Records of resource allocation/usage logs
Staff time sheets
Invoices
Receipts
By having these records on file, Nut & Co. confirmed that it was “compliance ready” — meaning if it was audited by the IRS, it could present documentation to show the progression of its R&D work, ultimately proving its R&D eligibility.
R&D Tax Credit Summary
Nut & Co. shows continuous improvements through research and development in the following areas:
Improvements to production time and efficiency
Improvements in reliability of products
Decrease in labor and production costs
Production innovation sourced from:
Internal ideas
Existing customers who have business needs which require new solutions
Nut & Co. eliminated uncertainty by:
Testing across all supported releases to determine reliability and user-friendliness
Experimentation with possible fixes until an adequate solution was determined
Therefore, Nut & Co. satisfies the four-part test and qualifies for both the Federal and State research and development tax credit. By filing both the federal and state research and development tax credits, Nut & Co. was able to obtain a significant amount of credit for tax years 2013-2016.
A Lincoln agricultural firm engaged KBKG to assist in the calculation of its R&D credit for federal and Nebraska purposes. The Company limited the scope of the study to include only the current year.
The Company qualified for the federal R&D Tax Credit of $105,000 and an additional $15,720 of state R&D Tax Credit in Nebraska.
| FEDERAL | NEBRASKA | |||||
| Year | Total QREs | Credit | Total QREs | Credit | ||
| Year 4 | $1.200.000,00 | $105.000,00 | $1.200.000,00 | $15.720,00 | ||
| Year 3 | $700.000,00 | N/A | $700.000,00 | N/A | ||
| Year 2 | $750.000,00 | N/A | $750.000,00 | N/A | ||
| Year 1 | $725.000,00 | N/A | $725.000,00 | N/A | ||
| Total | $3.375.000,00 | $105.000,00 | $3.375.000,00 | $15.720,00 | ||
Choose your state















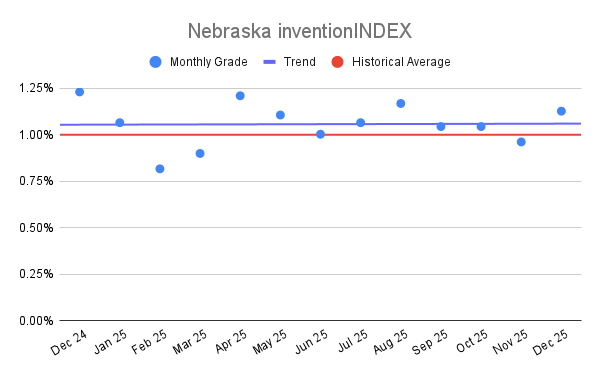 Nebraska inventionINDEX December 2025:[...]
Nebraska inventionINDEX December 2025:[...] Nebraska inventionINDEX Novemb
Nebraska inventionINDEX Novemb